Mostrar el registro sencillo del ítem
dc.contributor.author
Cobos, Carlos Jorge

dc.contributor.author
Croce, Adela Ester

dc.contributor.author
Luther, K.
dc.contributor.author
Sölter, L.
dc.contributor.author
Tellbach, E.
dc.contributor.author
Troe, J.
dc.date.available
2016-04-21T18:36:44Z
dc.date.issued
2013-10
dc.identifier.citation
Cobos, Carlos Jorge; Croce, Adela Ester; Luther, K.; Sölter, L.; Tellbach, E.; et al.; Experimental and modeling study of the reaction C2F4 (+M) = CF2 + CF2 (+M); American Chemical Society; Journal Of Physical Chemistry A; 117; 10-2013; 11420-11429
dc.identifier.issn
1089-5639
dc.identifier.uri
http://hdl.handle.net/11336/5321
dc.description.abstract
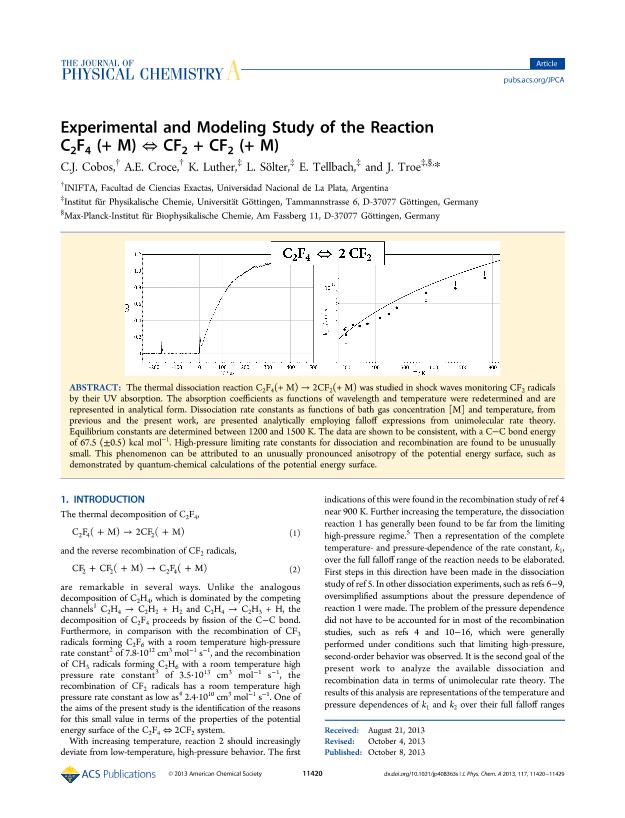
The thermal dissociation reaction C2F4(+ M) → 2CF2(+ M) was studied in shock waves monitoring CF2 radicals by their UV absorption. The absorption coefficients as functions of wavelength and temperature were redetermined and are represented in analytical form. Dissociation rate constants as functions of bath gas concentration [M] and temperature, from previous and the present work, are presented analytically employing falloff expressions from unimolecular rate theory. Equilibrium constants are determined between 1200 and 1500 K. The data are shown to be consistent, with a C–C bond energy of 67.5 (±0.5) kcal mol–1. High-pressure limiting rate constants for dissociation and recombination are found to be unusually small. This phenomenon can be attributed to an unusually pronounced anisotropy of the potential energy surface, such as demonstrated by quantum-chemical calculations of the potential energy surface.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
American Chemical Society

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject.classification
Físico-Química, Ciencia de los Polímeros, Electroquímica

dc.subject.classification
Ciencias Químicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Experimental and modeling study of the reaction C2F4 (+M) = CF2 + CF2 (+M)
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2016-05-06 15:52:43.262787-03
dc.journal.volume
117
dc.journal.pagination
11420-11429
dc.journal.pais
Estados Unidos

dc.journal.ciudad
Washington
dc.description.fil
Fil: Cobos, Carlos Jorge. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico la Plata. Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas; Argentina. Universidad Nacional de La Plata; Argentina
dc.description.fil
Fil: Croce, Adela Ester. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico la Plata. Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas; Argentina. Universidad Nacional de La Plata; Argentina
dc.description.fil
Fil: Luther, K.. Universität Göttingen. Institut für Physikalische Chemie; Alemania
dc.description.fil
Fil: Sölter, L.. Universität Göttingen. Institut für Physikalische Chemie; Alemania
dc.description.fil
Fil: Tellbach, E.. Universität Göttingen. Institut für Physikalische Chemie; Alemania
dc.description.fil
Fil: Troe, J.. Universität Göttingen. Institut für Physikalische Chemie; Alemania. Max-Planck-Institut für Biophysikalische Chemie; Alemania
dc.journal.title
Journal Of Physical Chemistry A

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/http://pubs.acs.org/doi/abs/10.1021/jp408363s
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/10.1021/jp408363s
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org/10.1021/jp408363s
Archivos asociados