Artículo
Experimental and modeling study of the reaction C2F4 (+M) = CF2 + CF2 (+M)
Fecha de publicación:
10/2013
Editorial:
American Chemical Society
Revista:
Journal Of Physical Chemistry A
ISSN:
1089-5639
Idioma:
Inglés
Tipo de recurso:
Artículo publicado
Clasificación temática:
Resumen
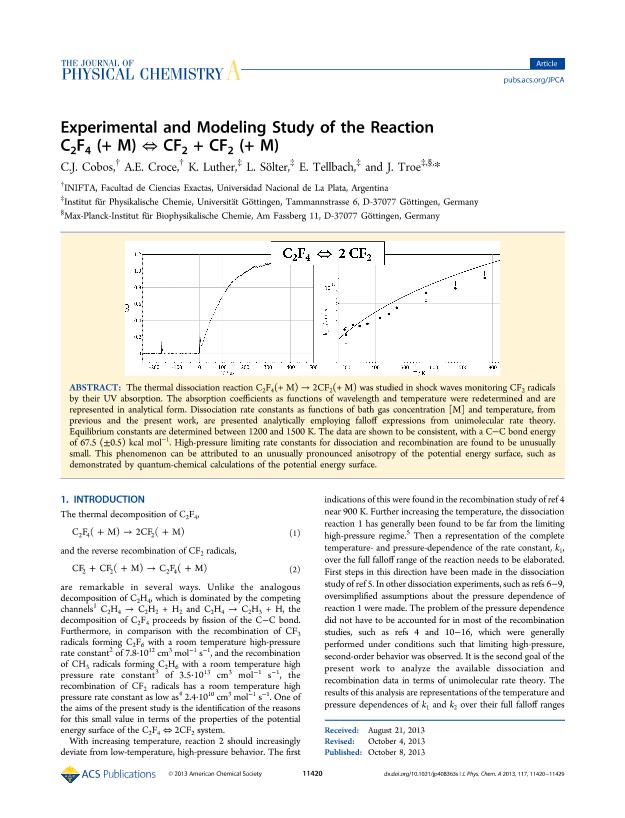
The thermal dissociation reaction C2F4(+ M) → 2CF2(+ M) was studied in shock waves monitoring CF2 radicals by their UV absorption. The absorption coefficients as functions of wavelength and temperature were redetermined and are represented in analytical form. Dissociation rate constants as functions of bath gas concentration [M] and temperature, from previous and the present work, are presented analytically employing falloff expressions from unimolecular rate theory. Equilibrium constants are determined between 1200 and 1500 K. The data are shown to be consistent, with a C–C bond energy of 67.5 (±0.5) kcal mol–1. High-pressure limiting rate constants for dissociation and recombination are found to be unusually small. This phenomenon can be attributed to an unusually pronounced anisotropy of the potential energy surface, such as demonstrated by quantum-chemical calculations of the potential energy surface.
Archivos asociados
Licencia
Identificadores
Colecciones
Articulos(INIFTA)
Articulos de INST.DE INV.FISICOQUIMICAS TEORICAS Y APLIC.
Articulos de INST.DE INV.FISICOQUIMICAS TEORICAS Y APLIC.
Articulos(SEDE CENTRAL)
Articulos de SEDE CENTRAL
Articulos de SEDE CENTRAL
Citación
Cobos, Carlos Jorge; Croce, Adela Ester; Luther, K.; Sölter, L.; Tellbach, E.; et al.; Experimental and modeling study of the reaction C2F4 (+M) = CF2 + CF2 (+M); American Chemical Society; Journal Of Physical Chemistry A; 117; 10-2013; 11420-11429
Compartir
Altmétricas