Mostrar el registro sencillo del ítem
dc.contributor.author
Borge, Mercedes

dc.contributor.author
Almejún, María Belén

dc.contributor.author
Podaza, Enrique Arturo

dc.contributor.author
Colado, Ana

dc.contributor.author
Fernández Grecco, Horacio
dc.contributor.author
Cabrejo, María
dc.contributor.author
Bezares, Raimundo F.

dc.contributor.author
Giordano, Mirta Nilda

dc.contributor.author
Gamberale, Romina

dc.date.available
2018-03-23T18:50:40Z
dc.date.issued
2015-01
dc.identifier.citation
Borge, Mercedes; Almejún, María Belén; Podaza, Enrique Arturo; Colado, Ana; Fernández Grecco, Horacio; et al.; Ibrutinib impairs the phagocytosis of rituximab-coated leukemic cells from chronic lymphocytic leukemia patients by human macrophages; Ferrata Storti Foundation; Haematologica; 100; 4; 1-2015; 1-3
dc.identifier.issn
0390-6078
dc.identifier.uri
http://hdl.handle.net/11336/39828
dc.description.abstract
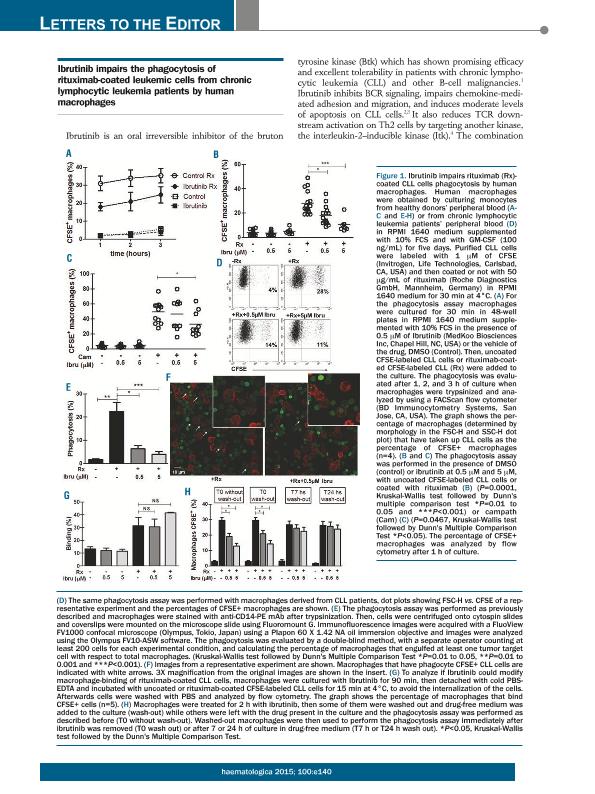
We have read with great interest the recent article of Kohrt, H.E. et al1 showing that Ibrutinib prevented NK cell mediated cytotoxicity of antibody-coated CLL cells in vitro. They also found that the concurrent treatment with Ibrutinib and rituximab or trastuzumab reduces the therapeutic efficacy of both anti-CD20 antibodies in a mouse model, while the sequential treatment with Ibrutinib and rituximab restored its anti-lymphoma activity. Since macrophages are the most important effector cells in CD20-directed cytotoxicity in murine models2,3 and they probably play a key role in human anti-CD20 therapy4,5, we determined whether Ibrutinib interferes the capacity of human macrophages to mediate phagocytosis of rituximab-coated CLL cells. To address this issue, macrophages differentiated from healthy peripheral blood monocytes were treated with or without Ibrutinib for 30 minutes and then cultured for 1, 2 or 3 hours with CFSE-labeled CLL cells or rituximab-coated CFSE-labeled CLL cells. Then, cells were tripsinized and the proportion of macrophages that have taken up CFSE-labeled CLL cells (CFSE+ macrophages) were scored by flow cytometry and verified using confocal microscopy, as previously described6. As expected, we found that the cultures with rituximab-coated CLL cells showed the highest percentage of CFSE+ macrophages, which increase in a time dependent manner (open circles in Figure 1A). Ibrutinib was able to reduce these values in all the times evaluated (solid circles in Figure 1A). Low percentages of CFSE+ macrophages were obtained in cultures with uncoated CLL cells, which were not modified by Ibrutinib (open and solid squares in Figure 1A). In addition, we found that Ibrutinib diminishes the percentage of CFSE+ macrophages in the cultures with rituximab-coated cells in a dose dependent manner (Figure 1B), which was not associated to a decreased viability of the macrophages (not shown). Moreover, the inhibitory effect of Ibrutinib was not limited to rituximab since comparable results were obtained when campath-coated CFSE-labeled CLL cells were employed (Figure 1C). Similar results were found when macrophages from CLL patients were used: mean±SE of the % of CFSE+ macrophages: 26.8 ± 2.1 vs, 17.3 ± 2.7 vs 10.8 ± 0.7 for rituximab-coated CFSE-labeled CLL cells alone, with 0.5μM or 5μM of Ibrutinib (n= 6). Representative dot plots are shown in Figure 1D. The results obtained by flow cytometry analysis were validated by confocal microscopy quantifying the number of macrophages that engulfed at least one tumor target cell (Figure 1E). A representative experiment is shown in Figure 1F. In addition, by performing a binding assay at 4oC, we confirmed that Ibrutinib did not reduce the binding of rituximab-coated CFSE-labeled CLL cells to macrophages (Figure 1G). Interestingly, while the presence of Ibrutinib during the assay impairs the phagocytosis of rituximab-coated CLL cells, when Ibrutinib was washed out, macrophages recovered their phagocytic capacity in a time-dependent manner (Figure 1H). In conclusion we found that the presence of Ibrutinib impairs the phagocytosis of rituximab-opsonized CLL cells by human macrophages, which was restored when the inhibitor was removed from the cultures. Our results, and those obtained by Kohrt et al1 suggest that the sequential administration of Ibrutinib followed by rituximab, and not the concurrent treatment of the patients with these agents, might enhance their anti-tumor activity in vivo.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
Ferrata Storti Foundation

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
Ibrutinib
dc.subject
Phagocytosis
dc.subject
Rituximab-Dependent
dc.subject.classification
Inmunología

dc.subject.classification
Medicina Básica

dc.subject.classification
CIENCIAS MÉDICAS Y DE LA SALUD

dc.title
Ibrutinib impairs the phagocytosis of rituximab-coated leukemic cells from chronic lymphocytic leukemia patients by human macrophages
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2018-02-28T14:14:16Z
dc.identifier.eissn
1592-8721
dc.journal.volume
100
dc.journal.number
4
dc.journal.pagination
1-3
dc.journal.pais
Italia

dc.journal.ciudad
Pavia
dc.description.fil
Fil: Borge, Mercedes. Universidad de Buenos Aires. Facultad de Medicina; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas. Instituto de Medicina Experimental. Academia Nacional de Medicina de Buenos Aires. Instituto de Medicina Experimental; Argentina
dc.description.fil
Fil: Almejún, María Belén. Universidad de Buenos Aires. Facultad de Medicina. Departamento de Microbiología. Cátedra de Microbiología, Parasitología e Inmunología; Argentina
dc.description.fil
Fil: Podaza, Enrique Arturo. Consejo Nacional de Investigaciones Científicas y Técnicas. Instituto de Medicina Experimental. Academia Nacional de Medicina de Buenos Aires. Instituto de Medicina Experimental; Argentina
dc.description.fil
Fil: Colado, Ana. Consejo Nacional de Investigaciones Científicas y Técnicas. Instituto de Medicina Experimental. Academia Nacional de Medicina de Buenos Aires. Instituto de Medicina Experimental; Argentina
dc.description.fil
Fil: Fernández Grecco, Horacio. Sanatorio Municipal Dr. Julio Méndez; Argentina
dc.description.fil
Fil: Cabrejo, María. Sanatorio Municipal Dr. Julio Méndez; Argentina
dc.description.fil
Fil: Bezares, Raimundo F.. Gobierno de la Ciudad de Buenos Aires. Hospital General de Agudos ; Argentina
dc.description.fil
Fil: Giordano, Mirta Nilda. Universidad de Buenos Aires. Facultad de Medicina; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas. Instituto de Medicina Experimental. Academia Nacional de Medicina de Buenos Aires. Instituto de Medicina Experimental; Argentina
dc.description.fil
Fil: Gamberale, Romina. Consejo Nacional de Investigaciones Científicas y Técnicas. Instituto de Medicina Experimental. Academia Nacional de Medicina de Buenos Aires. Instituto de Medicina Experimental; Argentina. Universidad de Buenos Aires. Facultad de Medicina; Argentina
dc.journal.title
Haematologica

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/http://www.haematologica.org/content/100/4/e140.long
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4380736/
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org/10.3324/haematol.2014.119669
Archivos asociados