Mostrar el registro sencillo del ítem
dc.contributor.author
Saporito Magriñá, Christian Martín

dc.contributor.author
Musacco Sebio, Rosario Natalia

dc.contributor.author
Andrieux, Geoffroy
dc.contributor.author
Kook, Lucas
dc.contributor.author
Orrego, Manuel Tomás
dc.contributor.author
Tuttolomondo, María Victoria

dc.contributor.author
Desimone, Martín Federico

dc.contributor.author
Boerries, Melanie
dc.contributor.author
Borner, Christoph
dc.contributor.author
Repetto, Marisa Gabriela

dc.date.available
2019-10-22T20:51:00Z
dc.date.issued
2018-12-03
dc.identifier.citation
Saporito Magriñá, Christian Martín; Musacco Sebio, Rosario Natalia; Andrieux, Geoffroy; Kook, Lucas; Orrego, Manuel Tomás; et al.; Copper-induced cell death and the protective role of glutathione: The implication of impaired protein folding rather than oxidative stress; Royal Society of Chemistry; Metallomics; 10; 12; 3-12-2018; 1743-1754
dc.identifier.issn
1756-5901
dc.identifier.uri
http://hdl.handle.net/11336/87018
dc.description.abstract
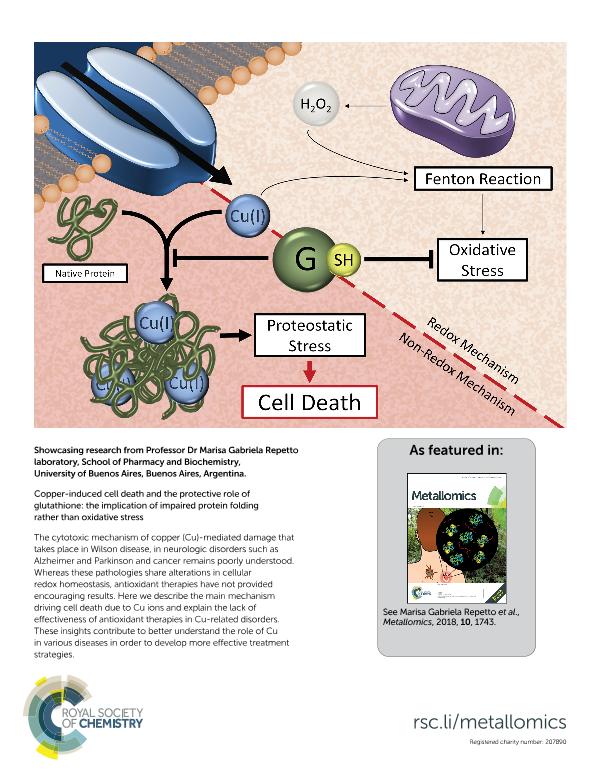
Copper (Cu) is a bioelement essential for a myriad of enzymatic reactions, which when present in high concentration leads to cytotoxicity. Whereas Cu toxicity is usually assumed to originate from the metal's ability to enhance lipid peroxidation, the role of oxidative stress has remained uncertain since no antioxidant therapy has ever been effective. Here we show that Cu overload induces cell death independently of the metal's ability to oxidize the intracellular milieu. In fact, cells neither lose control of their thiol homeostasis until briefly before the onset of cell death, nor trigger a consistent antioxidant response. As expected, glutathione (GSH) protects the cell from Cu-mediated cytotoxicity but, surprisingly, fully independent of its reactive thiol. Moreover, the oxidation state of extracellular Cu is irrelevant as cells accumulate the metal as cuprous ions. We provide evidence that cell death is driven by the interaction of cuprous ions with proteins which impairs protein folding and promotes aggregation. Consequently, cells mostly react to Cu by mounting a heat shock response and trying to restore protein homeostasis. The protective role of GSH is based on the binding of cuprous ions, thus preventing the metal interaction with proteins. Due to the high intracellular content of GSH, it is depleted near the Cu entry site, and hence Cu can interact with proteins and cause aggregation and cytotoxicity immediately below the plasma membrane.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
Royal Society of Chemistry

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
COPPER
dc.subject
OXIDATIVE STRESS
dc.subject
ANTIOXIDANT
dc.subject.classification
Bioquímica y Biología Molecular

dc.subject.classification
Ciencias Biológicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Copper-induced cell death and the protective role of glutathione: The implication of impaired protein folding rather than oxidative stress
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2019-10-16T20:35:38Z
dc.journal.volume
10
dc.journal.number
12
dc.journal.pagination
1743-1754
dc.journal.pais
Reino Unido

dc.journal.ciudad
Cambridge
dc.description.fil
Fil: Saporito Magriñá, Christian Martín. Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Musacco Sebio, Rosario Natalia. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina. Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica; Argentina
dc.description.fil
Fil: Andrieux, Geoffroy. German Cancer Research Center; Alemania. Universität Freiburg Im Breisgau; Alemania
dc.description.fil
Fil: Kook, Lucas. Universität Freiburg Im Breisgau; Alemania
dc.description.fil
Fil: Orrego, Manuel Tomás. Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica; Argentina
dc.description.fil
Fil: Tuttolomondo, María Victoria. Consejo Nacional de Investigaciones Científicas y Técnicas. Oficina de Coordinación Administrativa Houssay. Instituto de Química y Metabolismo del Fármaco. Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Instituto de Química y Metabolismo del Fármaco; Argentina. Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Departamento de Química Analítica y Fisicoquímica. Cátedra de Química Analítica Instrumental; Argentina
dc.description.fil
Fil: Desimone, Martín Federico. Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Departamento de Química Analítica y Fisicoquímica. Cátedra de Química Analítica Instrumental; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas. Oficina de Coordinación Administrativa Houssay. Instituto de Química y Metabolismo del Fármaco. Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Instituto de Química y Metabolismo del Fármaco; Argentina
dc.description.fil
Fil: Boerries, Melanie. Universität Freiburg Im Breisgau; Alemania
dc.description.fil
Fil: Borner, Christoph. Universität Freiburg Im Breisgau; Alemania
dc.description.fil
Fil: Repetto, Marisa Gabriela. Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Departamento de Química Analítica y Fisicoquímica. Cátedra de Química General e Inorgánica; Argentina. Universidad de Buenos Aires; Argentina
dc.journal.title
Metallomics

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org/10.1039/c8mt00182k
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/https://pubs.rsc.org/en/content/articlelanding/2018/MT/C8MT00182K#!divAbstract
Archivos asociados