Artículo
Cytochrome P450-Catalyzed Regio- and Stereoselective Phenol Coupling of Fungal Natural Products
Fecha de publicación:
09/2015
Editorial:
American Chemical Society
Revista:
Journal of the American Chemical Society
ISSN:
0002-7863
Idioma:
Inglés
Tipo de recurso:
Artículo publicado
Clasificación temática:
Resumen
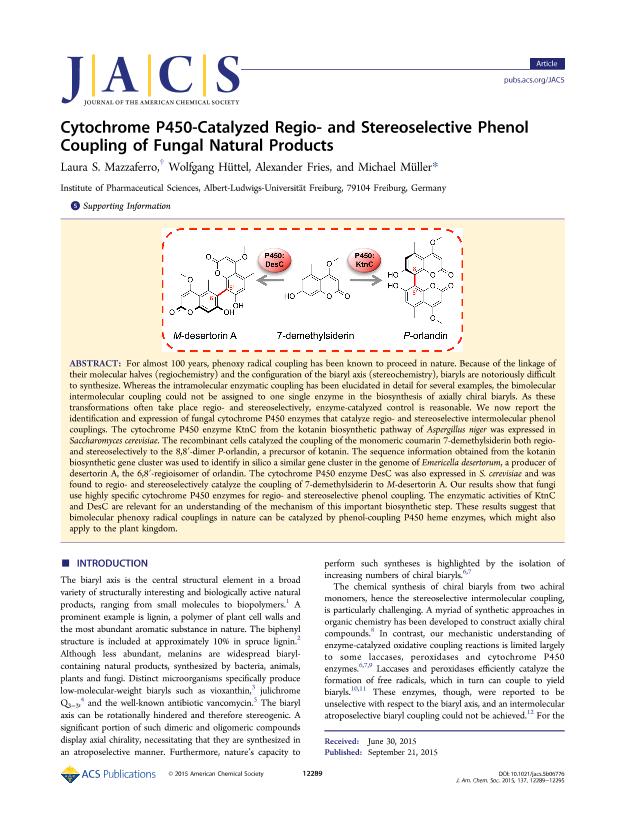
For almost 100 years, phenoxy radical coupling has been known to proceed in nature. Because of the linkage of their molecular halves (regiochemistry) and the configuration of the biaryl axis (stereochemistry), biaryls are notoriously difficult to synthesize. Whereas the intramolecular enzymatic coupling has been elucidated in detail for several examples, the bimolecular intermolecular coupling could not be assigned to one single enzyme in the biosynthesis of axially chiral biaryls. As these transformations often take place regio- and stereoselectively, enzyme-catalyzed control is reasonable. We now report the identification and expression of fungal cytochrome P450 enzymes that catalyze regio- and stereoselective intermolecular phenol couplings. The cytochrome P450 enzyme KtnC from the kotanin biosynthetic pathway of Aspergillus Niger was expressed in Saccharomyces cerevisiae. The recombinant cells catalyzed the coupling of the monomeric coumarin 7-demethylsiderin both regio- and stereoselectively to the 8,8′-dimer P-orlandin, a precursor of kotanin. The sequence information obtained from the kotanin biosynthetic gene cluster was used to identify in silico a similar gene cluster in the genome of Emericella desertorum, a producer of desertorin A, the 6,8′-regioisomer of orlandin. The cytochrome P450 enzyme DesC was also expressed in S. cerevisiae and was found to regio- and stereoselectively catalyze the coupling of 7-demethylsiderin to M-desertorin A. Our results show that fungi use highly specific cytochrome P450 enzymes for regio- and stereoselective phenol coupling. The enzymatic activities of KtnC and DesC are relevant for an understanding of the mechanism of this important biosynthetic step. These results suggest that bimolecular phenoxy radical couplings in nature can be catalyzed by phenol-coupling P450 heme enzymes, which might also apply to the plant kingdom.
Palabras clave:
Bicoumarin Synthase
,
Biaryls
,
Atropisomers
,
Biocatalysis
,
Asymmetric Synthesis
Archivos asociados
Licencia
Identificadores
Colecciones
Articulos(INCITAP)
Articulos de INST.D/CS D/L/TIERRA Y AMBIENTALES D/L/PAMPA
Articulos de INST.D/CS D/L/TIERRA Y AMBIENTALES D/L/PAMPA
Citación
Mazzaferro, Laura; Hüttel, Wolfgang; Fries, Alexander; Müller, Michael; Cytochrome P450-Catalyzed Regio- and Stereoselective Phenol Coupling of Fungal Natural Products; American Chemical Society; Journal of the American Chemical Society; 137; 38; 9-2015; 12289-12295
Compartir
Altmétricas