Mostrar el registro sencillo del ítem
dc.contributor.author
Moreno Betancourt, Angelica

dc.contributor.author
Bava, Yanina Belén

dc.contributor.author
Berrueta Martinez, Yanina

dc.contributor.author
Erben, Mauricio Federico

dc.contributor.author
Cavasso Filho Reinaldo L.
dc.contributor.author
Della Védova, Carlos Omar

dc.contributor.author
Romano, Rosana Mariel

dc.date.available
2018-12-05T15:30:16Z
dc.date.issued
2015-07
dc.identifier.citation
Moreno Betancourt, Angelica; Bava, Yanina Belén; Berrueta Martinez, Yanina; Erben, Mauricio Federico; Cavasso Filho Reinaldo L.; et al.; Photofragmentation Mechanisms of Chlorosulfonyl Isocyanate, ClSO2NCO, Excited with Synchrotron Radiation between 12 and 550 eV; American Chemical Society; Journal of Physical Chemistry A; 119; 29; 7-2015; 8021-8030
dc.identifier.issn
1089-5639
dc.identifier.uri
http://hdl.handle.net/11336/65858
dc.description.abstract
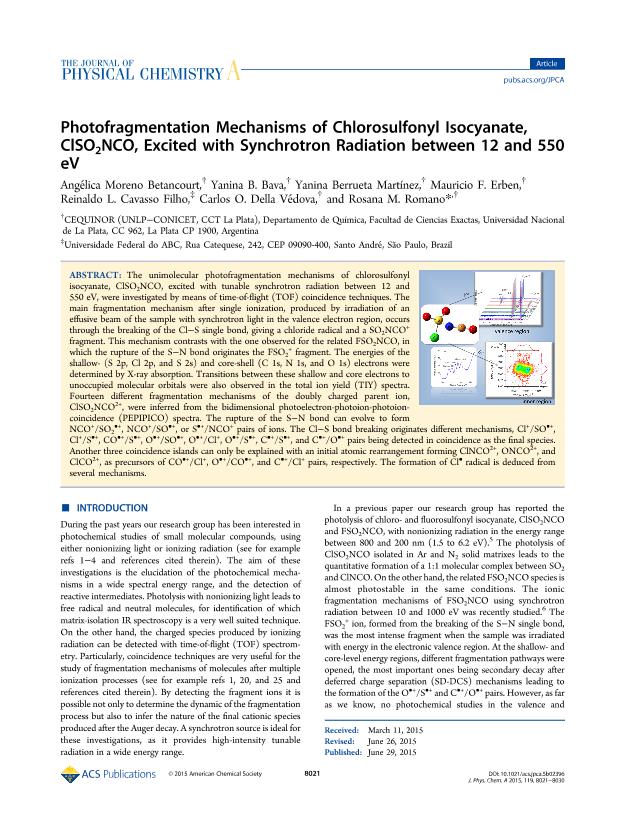
The unimolecular photofragmentation mechanisms of chlorosulfonyl isocyanate, ClSO2NCO, excited with tunable synchrotron radiation between 12 and 550 eV, were investigated by means of time-of-flight (TOF) coincidence techniques. The main fragmentation mechanism after single ionization, produced by irradiation of an effusive beam of the sample with synchrotron light in the valence electron region, occurs through the breaking of the Cl-S single bond, giving a chloride radical and a SO2NCO+ fragment. This mechanism contrasts with the one observed for the related FSO2NCO, in which the rupture of the S-N bond originates the FSO2+ fragment. The energies of the shallow- (S 2p, Cl 2p, and S 2s) and core-shell (C 1s, N 1s, and O 1s) electrons were determined by X-ray absorption. Transitions between these shallow and core electrons to unoccupied molecular orbitals were also observed in the total ion yield (TIY) spectra. Fourteen different fragmentation mechanisms of the doubly charged parent ion, ClSO2NCO2+, were inferred from the bidimensional photoelectron-photoion-photoion-coincidence (PEPIPICO) spectra. The rupture of the S-N bond can evolve to form NCO+/SO2•+, NCO+/SO•+, or S•+/NCO+ pairs of ions. The Cl-S bond breaking originates different mechanisms, Cl+/SO•+, Cl+/S•+, CO•+/S•+, O•+/SO•+, O•+/Cl+, O•+/S•+, C•+/S•+, and C•+/O•+ pairs being detected in coincidence as the final species. Another three coincidence islands can only be explained with an initial atomic rearrangement forming ClNCO2+, ONCO2+, and ClCO2+, as precursors of CO•+/Cl+, O•+/CO•+, and C•+/Cl+ pairs, respectively. The formation of Cl• radical is deduced from several mechanisms. (Figure Presented).
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
American Chemical Society

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
Photofragmentation
dc.subject
Synchrotron
dc.subject.classification
Otras Ciencias Químicas

dc.subject.classification
Ciencias Químicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Photofragmentation Mechanisms of Chlorosulfonyl Isocyanate, ClSO2NCO, Excited with Synchrotron Radiation between 12 and 550 eV
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2018-10-16T18:29:08Z
dc.journal.volume
119
dc.journal.number
29
dc.journal.pagination
8021-8030
dc.journal.pais
Estados Unidos

dc.journal.ciudad
Washington
dc.description.fil
Fil: Moreno Betancourt, Angelica. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - La Plata. Centro de Química Inorgánica "Dr. Pedro J. Aymonino". Universidad Nacional de La Plata. Facultad de Ciencias Exactas. Centro de Química Inorgánica "Dr. Pedro J. Aymonino"; Argentina
dc.description.fil
Fil: Bava, Yanina Belén. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - La Plata. Centro de Química Inorgánica "Dr. Pedro J. Aymonino". Universidad Nacional de La Plata. Facultad de Ciencias Exactas. Centro de Química Inorgánica "Dr. Pedro J. Aymonino"; Argentina
dc.description.fil
Fil: Berrueta Martinez, Yanina. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - La Plata. Centro de Química Inorgánica "Dr. Pedro J. Aymonino". Universidad Nacional de La Plata. Facultad de Ciencias Exactas. Centro de Química Inorgánica "Dr. Pedro J. Aymonino"; Argentina
dc.description.fil
Fil: Erben, Mauricio Federico. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - La Plata. Centro de Química Inorgánica "Dr. Pedro J. Aymonino". Universidad Nacional de La Plata. Facultad de Ciencias Exactas. Centro de Química Inorgánica "Dr. Pedro J. Aymonino"; Argentina
dc.description.fil
Fil: Cavasso Filho Reinaldo L.. Universidade Federal do ABC; Brasil
dc.description.fil
Fil: Della Védova, Carlos Omar. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - La Plata. Centro de Química Inorgánica "Dr. Pedro J. Aymonino". Universidad Nacional de La Plata. Facultad de Ciencias Exactas. Centro de Química Inorgánica "Dr. Pedro J. Aymonino"; Argentina
dc.description.fil
Fil: Romano, Rosana Mariel. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - La Plata. Centro de Química Inorgánica "Dr. Pedro J. Aymonino". Universidad Nacional de La Plata. Facultad de Ciencias Exactas. Centro de Química Inorgánica "Dr. Pedro J. Aymonino"; Argentina
dc.journal.title
Journal of Physical Chemistry A

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/http://pubs.acs.org/doi/abs/10.1021/acs.jpca.5b02396
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/https://dx.doi.org/10.1021/acs.jpca.5b02396
Archivos asociados