Mostrar el registro sencillo del ítem
dc.contributor.author
Calderón Segovia, Matías Francisco

dc.contributor.author
Zelaya, Maria Eugenia

dc.contributor.author
Benitez, Guillermo Alfredo

dc.contributor.author
Schilardi, Patricia Laura

dc.contributor.author
Hernandez Creus, Alberto
dc.contributor.author
Gonzalez Orive, Alejandro
dc.contributor.author
Salvarezza, Roberto Carlos

dc.contributor.author
Ibañez, Francisco Javier

dc.date.available
2016-03-18T19:00:24Z
dc.date.issued
2013-03-21
dc.identifier.citation
Calderón Segovia, Matías Francisco; Zelaya, Maria Eugenia; Benitez, Guillermo Alfredo; Schilardi, Patricia Laura; Hernandez Creus, Alberto; et al.; New Findings for the Composition and Structure of Ni Nanoparticles Protected with Organomercaptan Molecules; American Chemical Society; Langmuir; 29; 15; 21-3-2013; 4670-4678
dc.identifier.issn
0743-7463
dc.identifier.uri
http://hdl.handle.net/11336/4865
dc.description.abstract
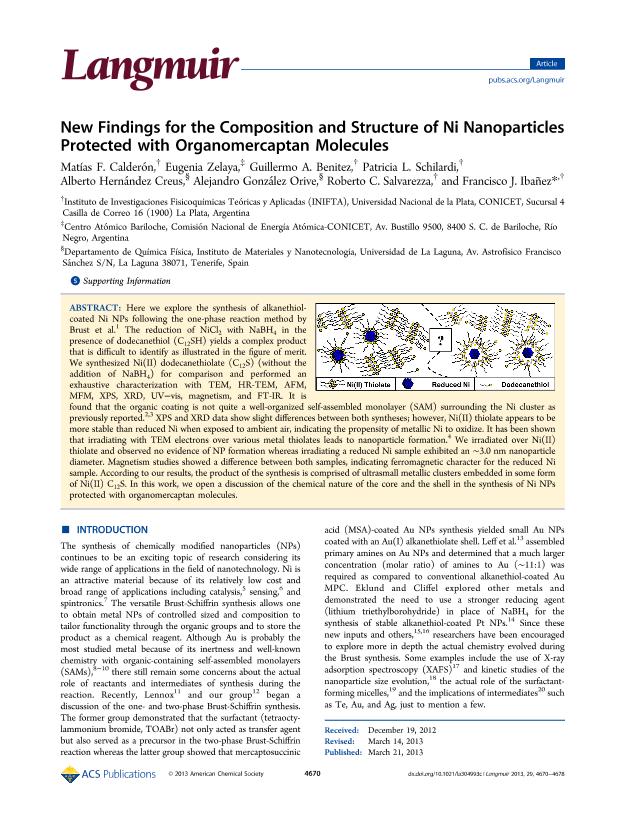
Here we explore the synthesis of alkanethiolcoated Ni NPs following the one-phase reaction method by Brust et al.1 The reduction of NiCl2 with NaBH4 in the presence of dodecanethiol (C12SH) yields a complex product that is difficult to identify as illustrated in the figure of merit. We synthesized Ni(II) dodecanethiolate (C12S) (without the addition of NaBH4) for comparison and performed an exhaustive characterization with TEM, HR-TEM, AFM, MFM, XPS, XRD, UV−vis, magnetism, and FT-IR. It is found that the organic coating is not quite a well-organized self-assembled monolayer (SAM) surrounding the Ni cluster as previously reported.2,3 XPS and XRD data show slight differences between both syntheses; however, Ni(II) thiolate appears to be more stable than reduced Ni when exposed to ambient air, indicating the propensity of metallic Ni to oxidize. It has been shown that irradiating with TEM electrons over various metal thiolates leads to nanoparticle formation.4 We irradiated over Ni(II) thiolate and observed no evidence of NP formation whereas irradiating a reduced Ni sample exhibited an ∼3.0 nm nanoparticle diameter. Magnetism studies showed a difference between both samples, indicating ferromagnetic character for the reduced Ni sample. According to our results, the product of the synthesis is comprised of ultrasmall metallic clusters embedded in some form of Ni(II) C12S. In this work, we open a discussion of the chemical nature of the core and the shell in the synthesis of Ni NPs protected with organomercaptan molecules.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
American Chemical Society

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
Alkanethiol
dc.subject
Ni Nanoparticles
dc.subject
Characterization
dc.subject
Ni Cluster
dc.subject
Ni(Ii) Thiolates
dc.subject.classification
Físico-Química, Ciencia de los Polímeros, Electroquímica

dc.subject.classification
Ciencias Químicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
New Findings for the Composition and Structure of Ni Nanoparticles Protected with Organomercaptan Molecules
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2016-03-30 10:35:44.97925-03
dc.journal.volume
29
dc.journal.number
15
dc.journal.pagination
4670-4678
dc.journal.pais
Estados Unidos

dc.journal.ciudad
Washington
dc.description.fil
Fil: Calderón Segovia, Matías Francisco. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico la Plata. Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas; Argentina
dc.description.fil
Fil: Zelaya, Maria Eugenia. Comisión Nacional de Energía Atómica. Gerencia del Area de Investigación y Aplicaciones No Nucleares. Gerencia de Física (Centro Atómico Bariloche); Argentina
dc.description.fil
Fil: Benitez, Guillermo Alfredo. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico la Plata. Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas; Argentina
dc.description.fil
Fil: Schilardi, Patricia Laura. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico la Plata. Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas; Argentina
dc.description.fil
Fil: Hernandez Creus, Alberto. Universidad de La Laguna, Instituto de Materiales y Nanotecnología, Departamento de Química Física; España
dc.description.fil
Fil: Gonzalez Orive, Alejandro. Universidad de La Laguna, Instituto de Materiales y Nanotecnología, Departamento de Química Física; España
dc.description.fil
Fil: Salvarezza, Roberto Carlos. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico la Plata. Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas; Argentina
dc.description.fil
Fil: Ibañez, Francisco Javier. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico la Plata. Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas; Argentina
dc.journal.title
Langmuir

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/10.1021/la304993c
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org//10.1021/la304993c
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/http://pubs.acs.org/doi/abs/10.1021/la304993c
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/pmid/23517502
Archivos asociados