Mostrar el registro sencillo del ítem
dc.contributor.author
Peirone, Silvina Anahí

dc.contributor.author
Nieto, Jorge Daniel

dc.contributor.author
Cometto, Pablo Marcelo

dc.contributor.author
Barbosa, Thaís da Silva
dc.contributor.author
Bauerfeldt, Glauco Favilla
dc.contributor.author
Arbilla, Graciela
dc.contributor.author
Lane, Silvia Irene

dc.date.available
2018-05-08T15:17:04Z
dc.date.issued
2015-03-18
dc.identifier.citation
Peirone, Silvina Anahí; Nieto, Jorge Daniel; Cometto, Pablo Marcelo; Barbosa, Thaís da Silva; Bauerfeldt, Glauco Favilla; et al.; Comparative kinetics of the 3-Buten-1-ol and 1-Butene reactions with OH radicals: a density functional theory/RRKM investigation; American Chemical Society; Journal of Physical Chemistry A; 119; 13; 18-3-2015; 3171-3180
dc.identifier.issn
1089-5639
dc.identifier.uri
http://hdl.handle.net/11336/44419
dc.description.abstract
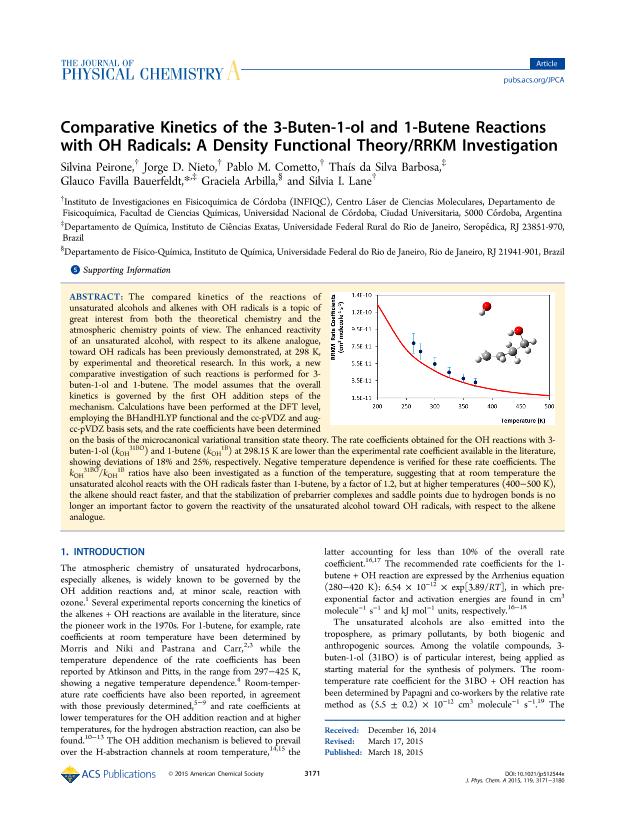
The compared kinetics of the reactions of unsaturated alcohols and alkenes with OH radicals is a topic of great interest from both the theoretical chemistry and the atmospheric chemistry points of view. The enhanced reactivity of an unsaturated alcohol, with respect to its alkene analogue, toward OH radicals has been previously demonstrated, at 298 K, by experimental and theoretical research. In this work, a new comparative investigation of such reactions is performed for 3-buten-1-ol and 1-butene. The model assumes that the overall kinetics is governed by the first OH addition steps of the mechanism. Calculations have been performed at the DFT level, employing the BHandHLYP functional and the cc-pVDZ and aug-cc-pVDZ basis sets, and the rate coefficients have been determined on the basis of the microcanonical variational transition state theory. The rate coefficients obtained for the OH reactions with 3-buten-1-ol (kOH31BO) and 1-butene (kOH1B) at 298.15 K are lower than the experimental rate coefficient available in the literature, showing deviations of 18% and 25%, respectively. Negative temperature dependence is verified for these rate coefficients. The kOH31BO/kOH1B ratios have also been investigated as a function of the temperature, suggesting that at room temperature the unsaturated alcohol reacts with the OH radicals faster than 1-butene, by a factor of 1.2, but at higher temperatures (400–500 K), the alkene should react faster, and that the stabilization of prebarrier complexes and saddle points due to hydrogen bonds is no longer an important factor to govern the reactivity of the unsaturated alcohol toward OH radicals, with respect to the alkene analogue.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
American Chemical Society

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
Kinetics Comparative
dc.subject
Rrkm
dc.subject
3-Buten-1-Ol
dc.subject
1-Butene
dc.subject.classification
Otras Ciencias Químicas

dc.subject.classification
Ciencias Químicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Comparative kinetics of the 3-Buten-1-ol and 1-Butene reactions with OH radicals: a density functional theory/RRKM investigation
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2018-04-26T17:52:52Z
dc.identifier.eissn
1520-5215
dc.journal.volume
119
dc.journal.number
13
dc.journal.pagination
3171-3180
dc.journal.pais
Estados Unidos

dc.journal.ciudad
Washington
dc.description.fil
Fil: Peirone, Silvina Anahí. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - Córdoba. Instituto de Investigaciones en Físico-química de Córdoba. Universidad Nacional de Córdoba. Facultad de Ciencias Químicas. Instituto de Investigaciones en Físico-química de Córdoba; Argentina. Universidad Nacional de Córdoba. Rectorado. Centro Laser de Ciencias Moleculares; Argentina
dc.description.fil
Fil: Nieto, Jorge Daniel. Universidad Nacional de Córdoba. Rectorado. Centro Laser de Ciencias Moleculares; Argentina
dc.description.fil
Fil: Cometto, Pablo Marcelo. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - Córdoba. Instituto de Investigaciones en Físico-química de Córdoba. Universidad Nacional de Córdoba. Facultad de Ciencias Químicas. Instituto de Investigaciones en Físico-química de Córdoba; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - Córdoba. Centro de Investigaciones y Transferencia de Villa María. Universidad Nacional de Villa María. Centro de Investigaciones y Transferencia de Villa María; Argentina
dc.description.fil
Fil: Barbosa, Thaís da Silva. Universidade Federal Rural do Rio de Janeiro; Brasil
dc.description.fil
Fil: Bauerfeldt, Glauco Favilla. Universidade Federal Rural do Rio de Janeiro; Brasil
dc.description.fil
Fil: Arbilla, Graciela. Universidade Federal do Rio de Janeiro; Brasil
dc.description.fil
Fil: Lane, Silvia Irene. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - Córdoba. Instituto de Investigaciones en Físico-química de Córdoba. Universidad Nacional de Córdoba. Facultad de Ciencias Químicas. Instituto de Investigaciones en Físico-química de Córdoba; Argentina
dc.journal.title
Journal of Physical Chemistry A

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/https://pubs.acs.org/doi/10.1021/jp512544x
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org/10.1021/jp512544x
Archivos asociados