Mostrar el registro sencillo del ítem
dc.contributor.author
Montero, María de Los Angeles

dc.contributor.author
Fernandez, Jose Luis

dc.contributor.author
Gennero, Maria Rosa

dc.contributor.author
Chialvo, Abel Cesar

dc.date.available
2017-09-01T19:42:03Z
dc.date.issued
2013-11
dc.identifier.citation
Montero, María de Los Angeles; Fernandez, Jose Luis; Gennero, Maria Rosa; Chialvo, Abel Cesar; Kinetic Study of the Hydrogen Oxidation Reaction on Nanostructured Iridium Electrodes in Acid Solutions; Amer Chemical Soc Inc; Journal of Physical Chemistry C; 117; 48; 11-2013; 25269-25275
dc.identifier.issn
1932-7447
dc.identifier.uri
http://hdl.handle.net/11336/23486
dc.description.abstract
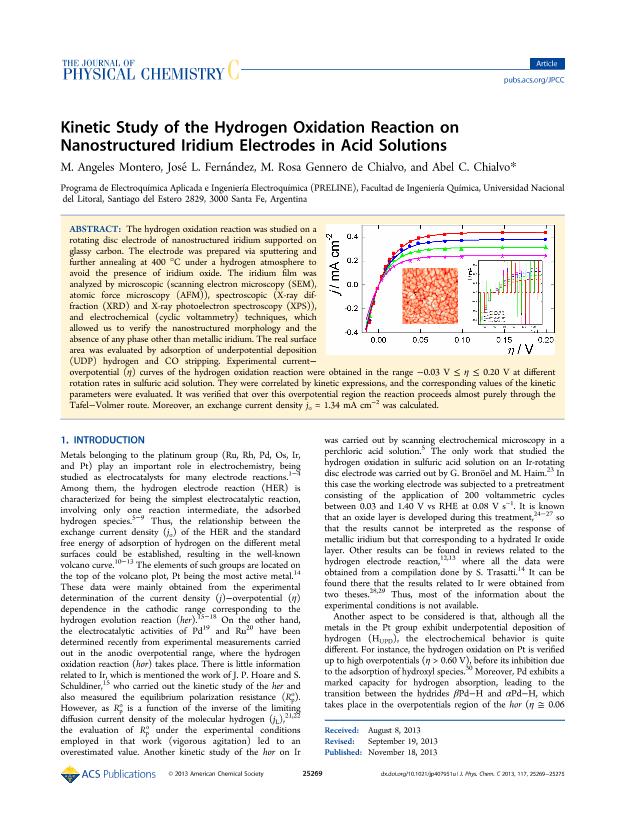
The hydrogen oxidation reaction was studied on a rotating disc electrode of nanostructured iridium supported on glassy carbon. The electrode was prepared via sputtering and further annealing at 400ºC under hydrogen atmosphere in order to avoid the presence of iridium oxide. The iridium film was analyzed by microscopic (SEM, AFM), spectroscopic (XRD and XPS) and electrochemical (cyclic voltammetry) techniques, which allowed to verify the nanostructured morphology and the absence of any phase other than metallic iridium. The real surface area was evaluated by adsorption of UPD hydrogen and CO stripping. Experimental current ? overpotential (E) curves of the hydrogen oxidation reaction were obtained in the range comprised between -0.03 V and 0.20 V at different rotation rates in sulphuric acid solution. They were correlated by kinetic expressions and the corresponding values of the kinetic parameters were evaluated. It was verified that over this overpotential region the reaction proceeds almost purely through the Tafel-Volmer route. Moreover, an exchange current density jo = 1.34 mA cm-2 was calculated.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
Amer Chemical Soc Inc

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
Iridium
dc.subject
Nanoparticles
dc.subject
Hydrogen Oxidation
dc.subject.classification
Otras Ciencias Químicas

dc.subject.classification
Ciencias Químicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Kinetic Study of the Hydrogen Oxidation Reaction on Nanostructured Iridium Electrodes in Acid Solutions
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2017-08-24T18:49:03Z
dc.journal.volume
117
dc.journal.number
48
dc.journal.pagination
25269-25275
dc.journal.pais
Estados Unidos

dc.description.fil
Fil: Montero, María de Los Angeles. Universidad Nacional del Litoral. Facultad de Ingeniería Química. Programa de Electroquímica Aplicada e Ingeniería Electroquímica; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Fernandez, Jose Luis. Universidad Nacional del Litoral. Facultad de Ingeniería Química. Programa de Electroquímica Aplicada e Ingeniería Electroquímica; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Gennero, Maria Rosa. Universidad Nacional del Litoral. Facultad de Ingeniería Química. Programa de Electroquímica Aplicada e Ingeniería Electroquímica; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Chialvo, Abel Cesar. Universidad Nacional del Litoral. Facultad de Ingeniería Química. Programa de Electroquímica Aplicada e Ingeniería Electroquímica; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.journal.title
Journal of Physical Chemistry C

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org/10.1021/jp407951u
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/http://pubs.acs.org/doi/abs/10.1021/jp407951u
Archivos asociados