Mostrar el registro sencillo del ítem
dc.contributor.author
Buzzi, Lucila Inés

dc.contributor.author
Segobia, Victoria Ayelén
dc.contributor.author
Rayes, Diego Hernán

dc.contributor.author
Castro, Olga Alejandra

dc.date.available
2022-07-19T19:05:16Z
dc.date.issued
2020-08
dc.identifier.citation
Buzzi, Lucila Inés; Segobia, Victoria Ayelén; Rayes, Diego Hernán; Castro, Olga Alejandra; The ER glycoprotein folding sensor UDP-Glc: glycoprotein glucosyltransferase is broadly expressed in C. elegans hermaphrodite; Caltech Library; microPublication Biology; 2020; 8-2020; 1-3
dc.identifier.issn
2578-9430
dc.identifier.uri
http://hdl.handle.net/11336/162584
dc.description.abstract
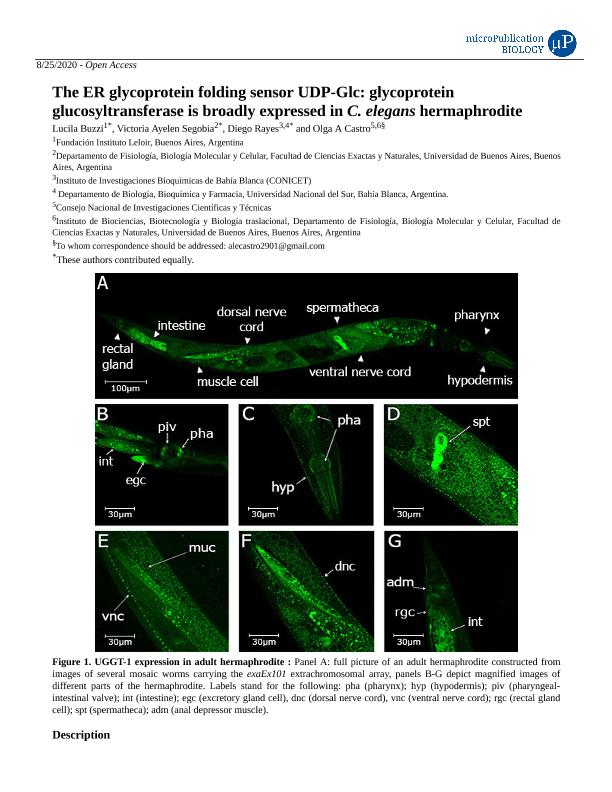
The endoplasmic reticulum (ER) uses an elaborate system called the ER quality control (QC) to monitor the proper folding of newly synthesized glycoproteins. The QC allows cells to differentiate between properly folded and misfolded proteins, allowing only those proteins which have acquired their native conformations to exit the ER and reach their final destinations. Alternatively, misfolded glycoproteins or incompletely formed glycoprotein complexes are translocated to the cytosol where they are finally degraded by proteasomes (Caramelo and Parodi 2007). The key element of this mechanism is the UDP-Glc: glycoprotein glucosyltransferase (UGGT) that functions as a folding sensor as it glucosylates exclusively those glycoproteins that have not acquired their native structures (Trombetta et al., 1989; Caramelo et al., 2003, 2004). Only vertebrates and Caenorhabditis genomes carry two uggt gene copies (uggt–1 and uggt–2) and phylogenetic inference showed that uggt genes went through independent duplications in Caenorhabditis and vertebrates. UGGT-1 retained canonical UGGT activity both in vertebrates and Caenorhabditis and vertebrate UGGT-2 underwent a specialization process. In Caenorhabditis, uggt-2 evolved by means of a putative neofunctionalization process in a non-redundant paralog and its biological function is still unknown (Caraballo et al., 2020; Buzzi et al.., 2011). Hence, UGGT-1 is the only protein engaged in monitoring the folding state of every glycoprotein in Caenorhabditis ER. To determine C. elegans UGGT-1’s body pattern expression we used fosmid recombineering technology (Tursun et al., 2009) to generate the Puggt-1::sl2::nls::gfp::unc-54 3’UTR transcriptional fusion reporter and established worm lines expressing this construct. UGGT-1 is expressed in the head, both in the pharynx, (corpus, isthmus and terminal bulb and buccal cavity) and in the pharyngeal intestinal valve. In the same image its expression is detected in the hypodermis and in the secretory gland (B and C). The somatic cells of the spermatheca express UGGT-1, but not the germline (D). Consistent with our previous findings (Buzzi et al.., 2011) UGGT-1 is widely expressed in the nervous system, both in ventral and dorsal nerve cords (E and F), as well as in the muscle cells as shown in (E-F) and in the anal depressor muscle (G). In the tail expression is also observed both in the rectal gland cell and the intestine.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
Caltech Library
dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by/2.5/ar/
dc.subject
GLYCOPROTEIN
dc.subject
GLUCOSYLTRANSFERASE
dc.subject
FOLDING SENSOR
dc.subject
C.ELEGANS
dc.subject.classification
Biología Celular, Microbiología

dc.subject.classification
Ciencias Biológicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
The ER glycoprotein folding sensor UDP-Glc: glycoprotein glucosyltransferase is broadly expressed in C. elegans hermaphrodite
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2022-07-15T14:49:48Z
dc.journal.volume
2020
dc.journal.pagination
1-3
dc.journal.pais
Estados Unidos

dc.journal.ciudad
Pasadena
dc.description.fil
Fil: Buzzi, Lucila Inés. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina. Fundación Instituto Leloir; Argentina
dc.description.fil
Fil: Segobia, Victoria Ayelén. Universidad de Buenos Aires. Facultad de Ciencias Exactas y Naturales. Departamento de Fisiología, Biología Molecular y Celular; Argentina
dc.description.fil
Fil: Rayes, Diego Hernán. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - Bahía Blanca. Instituto de Investigaciones Bioquímicas de Bahía Blanca. Universidad Nacional del Sur. Instituto de Investigaciones Bioquímicas de Bahía Blanca; Argentina. Universidad Nacional del Sur. Departamento de Biología, Bioquímica y Farmacia; Argentina
dc.description.fil
Fil: Castro, Olga Alejandra. Consejo Nacional de Investigaciones Científicas y Técnicas. Oficina de Coordinación Administrativa Ciudad Universitaria; Argentina. Universidad de Buenos Aires. Facultad de Ciencias Exactas y Naturales. Instituto de Biociencias, Biotecnología y Biología Traslacional; Argentina
dc.journal.title
microPublication Biology
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/https://www.micropublication.org/journals/biology/micropub-biology-000299
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org/10.17912/micropub.biology.000299.
Archivos asociados