Mostrar el registro sencillo del ítem
dc.contributor.author
Del Árbol, Nerea Ruiz
dc.contributor.author
Palacio, Irene
dc.contributor.author
Sánchez Sánchez, Carlos
dc.contributor.author
Otero Irurueta, Gonzalo
dc.contributor.author
Martínez, José I.
dc.contributor.author
Rodriguez, Luis Miguel

dc.contributor.author
Serrate, David
dc.contributor.author
Cossaro, Albano
dc.contributor.author
Lacovig, Paolo
dc.contributor.author
Lizzit, Silvano
dc.contributor.author
Verdini, Alberto
dc.contributor.author
Floreano, Luca
dc.contributor.author
Martín Gago, José Ángel

dc.contributor.author
López, María F.
dc.date.available
2021-11-12T10:55:46Z
dc.date.issued
2020-09
dc.identifier.citation
Del Árbol, Nerea Ruiz; Palacio, Irene; Sánchez Sánchez, Carlos; Otero Irurueta, Gonzalo; Martínez, José I.; et al.; Role of the Metal Surface on the Room Temperature Activation of the Alcohol and Amino Groups of p-Aminophenol; American Chemical Society; Journal of Physical Chemistry C; 124; 36; 9-2020; 19655-19665
dc.identifier.issn
1932-7447
dc.identifier.uri
http://hdl.handle.net/11336/146773
dc.description.abstract
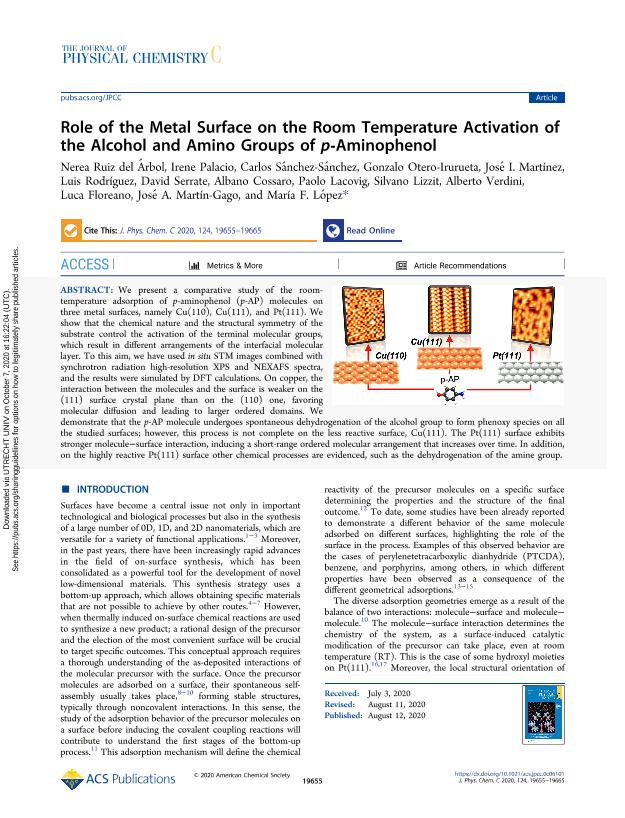
We present a comparative study of the room-temperature adsorption of p-aminophenol (p-AP) molecules on three metal surfaces, namely Cu(110), Cu(111), and Pt(111). We show that the chemical nature and the structural symmetry of the substrate control the activation of the terminal molecular groups, which result in different arrangements of the interfacial molecular layer. To this aim, we have used in situ STM images combined with synchrotron radiation high-resolution XPS and NEXAFS spectra, and the results were simulated by DFT calculations. On copper, the interaction between the molecules and the surface is weaker on the (111) surface crystal plane than on the (110) one, favoring molecular diffusion and leading to larger ordered domains. We demonstrate that the p-AP molecule undergoes spontaneous dehydrogenation of the alcohol group to form phenoxy species on all the studied surfaces; however, this process is not complete on the less reactive surface, Cu(111). The Pt(111) surface exhibits stronger molecule-surface interaction, inducing a short-range ordered molecular arrangement that increases over time. In addition, on the highly reactive Pt(111) surface other chemical processes are evidenced, such as the dehydrogenation of the amine group.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
American Chemical Society

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
aminophenol
dc.subject
surface science
dc.subject
STM
dc.subject.classification
Otras Ciencias Físicas

dc.subject.classification
Ciencias Físicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Role of the Metal Surface on the Room Temperature Activation of the Alcohol and Amino Groups of p-Aminophenol
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2021-10-20T18:20:13Z
dc.journal.volume
124
dc.journal.number
36
dc.journal.pagination
19655-19665
dc.journal.pais
Estados Unidos

dc.description.fil
Fil: Del Árbol, Nerea Ruiz. Instituto de Ciencia de Materiales de Madrid; España
dc.description.fil
Fil: Palacio, Irene. Instituto de Ciencia de Materiales de Madrid; España
dc.description.fil
Fil: Sánchez Sánchez, Carlos. Instituto de Ciencia de Materiales de Madrid; España
dc.description.fil
Fil: Otero Irurueta, Gonzalo. Universidade de Aveiro; Portugal
dc.description.fil
Fil: Martínez, José I.. Instituto de Ciencia de Materiales de Madrid; España
dc.description.fil
Fil: Rodriguez, Luis Miguel. Instituto de Ciencia de Materiales de Madrid; España. Consejo Nacional de Investigaciones Científicas y Técnicas. Oficina de Coordinación Administrativa Ciudad Universitaria. Unidad Ejecutora Instituto de Nanociencia y Nanotecnología. Unidad Ejecutora Instituto de Nanociencia y Nanotecnología - Nodo Bariloche | Comisión Nacional de Energía Atómica. Unidad Ejecutora Instituto de Nanociencia y Nanotecnología. Unidad Ejecutora Instituto de Nanociencia y Nanotecnología - Nodo Bariloche; Argentina
dc.description.fil
Fil: Serrate, David. Universidad de Zaragoza. Instituto de Ciencias de Materiales de Aragon; España
dc.description.fil
Fil: Cossaro, Albano. Università degli Studi di Trieste; Italia. Laboratorio Nazionale Tasc; Italia
dc.description.fil
Fil: Lacovig, Paolo. Elettra - Synchrotron Ligght Laboratory; Italia
dc.description.fil
Fil: Lizzit, Silvano. Elettra - Synchrotron Ligght Laboratory; Italia
dc.description.fil
Fil: Verdini, Alberto. Laboratorio Nazionale Tasc; Italia
dc.description.fil
Fil: Floreano, Luca. No especifíca;
dc.description.fil
Fil: Martín Gago, José Ángel. Instituto de Ciencia de Materiales de Madrid; España
dc.description.fil
Fil: López, María F.. Instituto de Ciencia de Materiales de Madrid; España
dc.journal.title
Journal of Physical Chemistry C

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org/10.1021/acs.jpcc.0c06101
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/https://pubs.acs.org/doi/10.1021/acs.jpcc.0c06101
Archivos asociados