Mostrar el registro sencillo del ítem
dc.contributor.author
Nielsen, Beatriz Elizabeth

dc.contributor.author
Stabile, Santiago Armando

dc.contributor.author
Vitale, Cristian Alejandro

dc.contributor.author
Bouzat, Cecilia Beatriz

dc.date.available
2021-10-07T14:38:10Z
dc.date.issued
2020-07-28
dc.identifier.citation
Nielsen, Beatriz Elizabeth; Stabile, Santiago Armando; Vitale, Cristian Alejandro; Bouzat, Cecilia Beatriz; Design, synthesis and functional evaluation of a novel series of phosphonate-functionalized 1,2,3-triazoles as positive allosteric modulators of α7 nicotinic acetylcholine receptors; American Chemical Society; ACS Chemical Neuroscience; 11; 17; 28-7-2020; 2688–2704
dc.identifier.issn
1948-7193
dc.identifier.uri
http://hdl.handle.net/11336/143117
dc.description.abstract
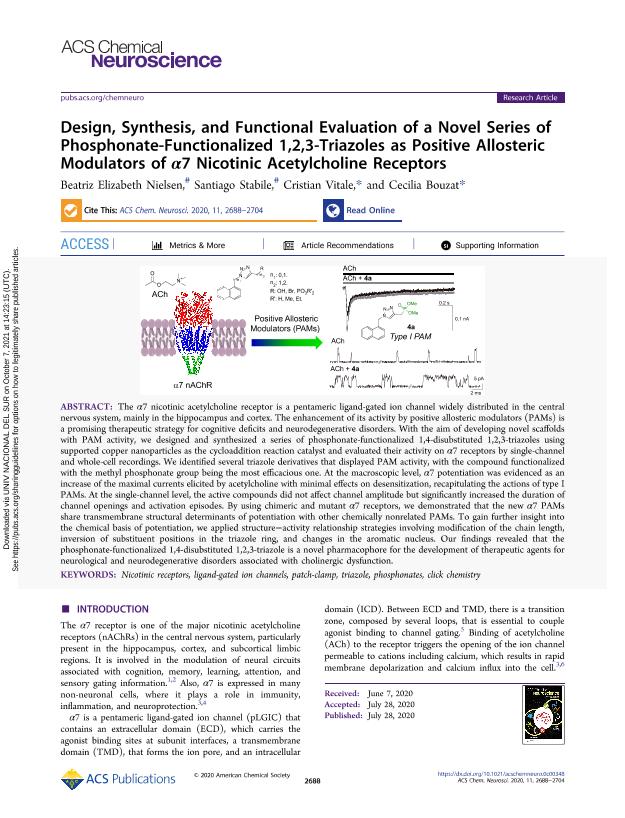
The α7 nicotinic acetylcholine receptor is a pentameric ligand-gated ion channel widely distributed in the central nervous system, mainly in hippocampus and cortex. The enhancement of its activity by positive allosteric modulators (PAMs) is a promising therapeutic strategy for cognitive deficits and neurodegenerative disorders. With the aim of developing novel scaffolds with PAM activity, we designed and synthesized a series of phosphonate-functionalized 1,4-disubstituted 1,2,3-triazoles using supported copper nanoparticles as cycloaddition reaction catalyst, and evaluated their activity on α7 receptors by single-channel and whole-cell recordings. We identified several triazole derivatives that displayed PAM activity, the compound functionalized with the methyl phosphonate group being the most efficacious one. At the macroscopic level, α7 potentiation was evidenced as an increase of the maximal currents elicited by acetylcholine with minimal effects on desensitization, recapitulating the actions of type I PAMs. At the single-channel level, the active compounds did not affect channel amplitude, but significantly increased the duration of channel openings and activation episodes. By using chimeric and mutant α7 receptors, we demonstrated that the new α7 PAMs share transmembrane structural determinants of potentiation with other chemically non-related PAMs. To gain further insight into the chemical basis of potentiation, we applied structure-activity relationship strategies involving modification of the chain length, inversion of substituent positions in the triazole ring and changes in the aromatic nucleus. Our findings revealed that the phosphonate-functionalized 1,4-disubstituted 1,2,3-triazole is a novel pharmacophore for the development of therapeutic agents for neurological and neurodegenerative disorders associated to cholinergic dysfunction.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
American Chemical Society
dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
NICOTINIC RECEPTORS
dc.subject
LIGAND-GATED ION CHANNELS
dc.subject
PATCH CLAMP
dc.subject
TRIAZOLE
dc.subject
PHOSPHONATES
dc.subject
CLICK CHEMISTRY
dc.subject.classification
Biofísica

dc.subject.classification
Ciencias Biológicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Design, synthesis and functional evaluation of a novel series of phosphonate-functionalized 1,2,3-triazoles as positive allosteric modulators of α7 nicotinic acetylcholine receptors
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2020-09-02T19:04:19Z
dc.journal.volume
11
dc.journal.number
17
dc.journal.pagination
2688–2704
dc.journal.pais
Estados Unidos

dc.journal.ciudad
Washington DC.
dc.description.fil
Fil: Nielsen, Beatriz Elizabeth. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - Bahía Blanca. Instituto de Investigaciones Bioquímicas de Bahía Blanca. Universidad Nacional del Sur. Instituto de Investigaciones Bioquímicas de Bahía Blanca; Argentina
dc.description.fil
Fil: Stabile, Santiago Armando. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - Bahía Blanca. Instituto de Química del Sur. Universidad Nacional del Sur. Departamento de Química. Instituto de Química del Sur; Argentina
dc.description.fil
Fil: Vitale, Cristian Alejandro. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - Bahía Blanca. Instituto de Química del Sur. Universidad Nacional del Sur. Departamento de Química. Instituto de Química del Sur; Argentina
dc.description.fil
Fil: Bouzat, Cecilia Beatriz. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - Bahía Blanca. Instituto de Investigaciones Bioquímicas de Bahía Blanca. Universidad Nacional del Sur. Instituto de Investigaciones Bioquímicas de Bahía Blanca; Argentina
dc.journal.title
ACS Chemical Neuroscience
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/https://doi.org/10.1021/acschemneuro.0c00348
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/https://pubs.acs.org/doi/10.1021/acschemneuro.0c00348
Archivos asociados