Mostrar el registro sencillo del ítem
dc.contributor.author
Barletta Roldan, Patricio German

dc.contributor.author
Franchini, Gisela Raquel

dc.contributor.author
Córsico, Betina

dc.contributor.author
Fernández Alberti, Sebastián

dc.date.available
2021-04-29T14:51:15Z
dc.date.issued
2019-07-31
dc.identifier.citation
Barletta Roldan, Patricio German; Franchini, Gisela Raquel; Córsico, Betina; Fernández Alberti, Sebastián; Fatty acid and retinol-binding protein: Unusual protein conformational and cavity changes dictated by ligand fluctuations; American Chemical Society; Journal of Chemical Information and Modeling; 59; 8; 31-7-2019; 3545-3555
dc.identifier.issn
1549-9596
dc.identifier.uri
http://hdl.handle.net/11336/131023
dc.description.abstract
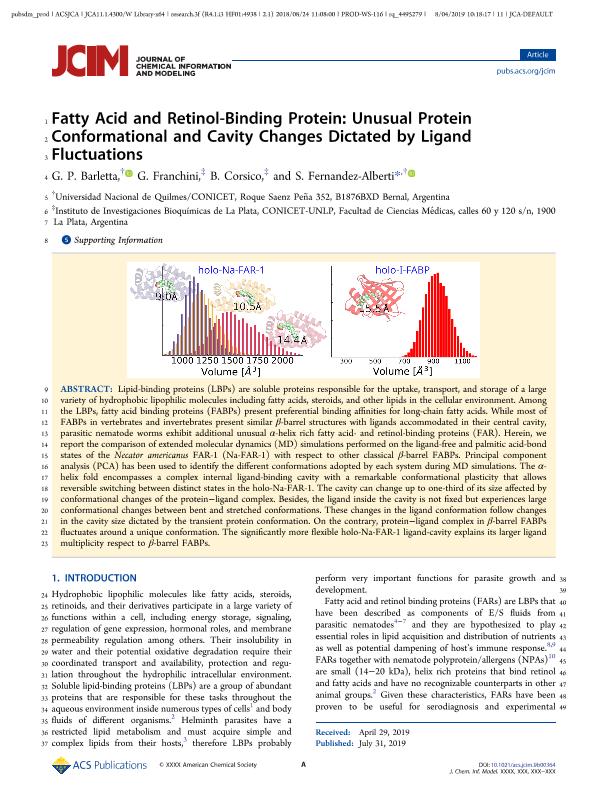
Lipid-binding proteins (LBPs) are soluble proteins responsible for the uptake, transport, and storage of a large variety of hydrophobic lipophilic molecules including fatty acids, steroids, and other lipids in the cellular environment. Among the LBPs, fatty acid binding proteins (FABPs) present preferential binding affinities for long-chain fatty acids. While most of FABPs in vertebrates and invertebrates present similar β-barrel structures with ligands accommodated in their central cavity, parasitic nematode worms exhibit additional unusual α-helix rich fatty acid- and retinol-binding proteins (FAR). Herein, we report the comparison of extended molecular dynamics (MD) simulations performed on the ligand-free and palmitic acid-bond states of the Necator americanus FAR-1 (Na-FAR-1) with respect to other classical β-barrel FABPs. Principal component analysis (PCA) has been used to identify the different conformations adopted by each system during MD simulations. The α-helix fold encompasses a complex internal ligand-binding cavity with a remarkable conformational plasticity that allows reversible switching between distinct states in the holo-Na-FAR-1. The cavity can change up to one-third of its size affected by conformational changes of the protein-ligand complex. Besides, the ligand inside the cavity is not fixed but experiences large conformational changes between bent and stretched conformations. These changes in the ligand conformation follow changes in the cavity size dictated by the transient protein conformation. On the contrary, protein-ligand complex in β-barrel FABPs fluctuates around a unique conformation. The significantly more flexible holo-Na-FAR-1 ligand-cavity explains its larger ligand multiplicity respect to β-barrel FABPs.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
American Chemical Society

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
LIPID
dc.subject
BINDING
dc.subject
MOLECULAR
dc.subject
DYNAMICS
dc.subject.classification
Biofísica

dc.subject.classification
Ciencias Biológicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Fatty acid and retinol-binding protein: Unusual protein conformational and cavity changes dictated by ligand fluctuations
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2021-04-22T20:11:10Z
dc.journal.volume
59
dc.journal.number
8
dc.journal.pagination
3545-3555
dc.journal.pais
Estados Unidos

dc.journal.ciudad
Washington
dc.description.fil
Fil: Barletta Roldan, Patricio German. Universidad Nacional de Quilmes. Departamento de Ciencia y Tecnología; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Franchini, Gisela Raquel. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - La Plata. Instituto de Investigaciones Bioquímicas de La Plata "Prof. Dr. Rodolfo R. Brenner". Universidad Nacional de la Plata. Facultad de Ciencias Médicas. Instituto de Investigaciones Bioquímicas de La Plata "Prof. Dr. Rodolfo R. Brenner"; Argentina
dc.description.fil
Fil: Córsico, Betina. Consejo Nacional de Investigaciones Científicas y Técnicas. Centro Científico Tecnológico Conicet - La Plata. Instituto de Investigaciones Bioquímicas de La Plata "Prof. Dr. Rodolfo R. Brenner". Universidad Nacional de la Plata. Facultad de Ciencias Médicas. Instituto de Investigaciones Bioquímicas de La Plata "Prof. Dr. Rodolfo R. Brenner"; Argentina
dc.description.fil
Fil: Fernández Alberti, Sebastián. Universidad Nacional de Quilmes. Departamento de Ciencia y Tecnología; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.journal.title
Journal of Chemical Information and Modeling

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org/10.1021/acs.jcim.9b00364
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/https://pubs.acs.org/doi/10.1021/acs.jcim.9b00364
Archivos asociados