Mostrar el registro sencillo del ítem
dc.contributor.author
Alves, Norma Roxana Carina

dc.contributor.author
Pecci, Adali

dc.contributor.author
Alvarez, Lautaro Damian

dc.date.available
2021-01-21T11:53:36Z
dc.date.issued
2019-11
dc.identifier.citation
Alves, Norma Roxana Carina; Pecci, Adali; Alvarez, Lautaro Damian; Structural Insights into the Ligand Binding Domain of the Glucocorticoid Receptor: A Molecular Dynamics Study; American Chemical Society; Journal of Chemical Information and Modeling; 60; 2; 11-2019; 794-804
dc.identifier.issn
1549-9596
dc.identifier.uri
http://hdl.handle.net/11336/123255
dc.description.abstract
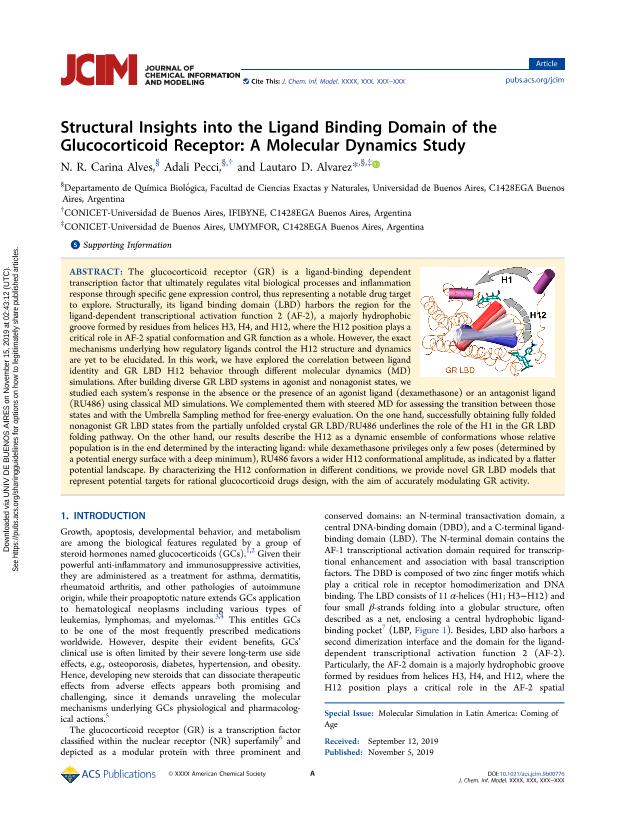
The glucocorticoid receptor (GR) is a ligand-binding dependent transcription factor that ultimately regulates vital biological processes and inflammation response through specific gene expression control, thus representing a notable drug target to explore. Structurally, its ligand binding domain (LBD) harbors the region for the ligand-dependent transcriptional activation function 2 (AF-2), a majorly hydrophobic groove formed by residues from helices H3, H4, and H12, where the H12 position plays a critical role in AF-2 spatial conformation and GR function as a whole. However, the exact mechanisms underlying how regulatory ligands control the H12 structure and dynamics are yet to be elucidated. In this work, we have explored the correlation between ligand identity and GR LBD H12 behavior through different molecular dynamics (MD) simulations. After building diverse GR LBD systems in agonist and nonagonist states, we studied each system?s response in the absence or the presence of an agonist ligand (dexamethasone) or an antagonist ligand (RU486) using classical MD simulations. We complemented them with steered MD for assessing the transition between those states and with the Umbrella Sampling method for free-energy evaluation. On the one hand, successfully obtaining fully folded nonagonist GR LBD states from the partially unfolded crystal GR LBD/RU486 underlines the role of the H1 in the GR LBD folding pathway. On the other hand, our results describe the H12 as a dynamic ensemble of conformations whose relative population is in the end determined by the interacting ligand: while dexamethasone privileges only a few poses (determined by a potential energy surface with a deep minimum), RU486 favors a wider H12 conformational amplitude, as indicated by a flatter potential landscape. By characterizing the H12 conformation in different conditions, we provide novel GR LBD models that represent potential targets for rational glucocorticoid drugs design, with the aim of accurately modulating GR activity.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
American Chemical Society

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
Glucocorticoid
dc.subject
Molecular mechanism
dc.subject
Enhaced sampled methods
dc.subject
ligand
dc.subject.classification
Biofísica

dc.subject.classification
Ciencias Biológicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Structural Insights into the Ligand Binding Domain of the Glucocorticoid Receptor: A Molecular Dynamics Study
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2020-12-04T18:12:29Z
dc.journal.volume
60
dc.journal.number
2
dc.journal.pagination
794-804
dc.journal.pais
Estados Unidos

dc.journal.ciudad
Washington
dc.description.fil
Fil: Alves, Norma Roxana Carina. Universidad de Buenos Aires. Facultad de Ciencias Exactas y Naturales. Departamento de Química Biológica; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Pecci, Adali. Consejo Nacional de Investigaciones Científicas y Técnicas. Oficina de Coordinación Administrativa Ciudad Universitaria. Instituto de Fisiología, Biología Molecular y Neurociencias. Universidad de Buenos Aires. Facultad de Ciencias Exactas y Naturales. Instituto de Fisiología, Biología Molecular y Neurociencias; Argentina. Universidad de Buenos Aires. Facultad de Ciencias Exactas y Naturales. Departamento de Química Biológica; Argentina
dc.description.fil
Fil: Alvarez, Lautaro Damian. Universidad de Buenos Aires. Facultad de Ciencias Exactas y Naturales. Departamento de Química Biológica; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas. Oficina de Coordinación Administrativa Ciudad Universitaria. Unidad de Microanálisis y Métodos Físicos en Química Orgánica. Universidad de Buenos Aires. Facultad de Ciencias Exactas y Naturales. Unidad de Microanálisis y Métodos Físicos en Química Orgánica; Argentina
dc.journal.title
Journal of Chemical Information and Modeling

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/https://pubs.acs.org/doi/10.1021/acs.jcim.9b00776
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/http://dx.doi.org/10.1021/acs.jcim.9b00776
Archivos asociados