Mostrar el registro sencillo del ítem
dc.contributor.author
Rodríguez Hernández, Beatriz

dc.contributor.author
Ondarse Alvarez, Dianelys

dc.contributor.author
Oldani, Andres Nicolas

dc.contributor.author
Martinez Mesa, Aliezer

dc.contributor.author
Uranga Pina, Llinersy
dc.contributor.author
Tretiak, Sergei

dc.contributor.author
Fernández Alberti, Sebastián

dc.date.available
2020-11-04T13:41:53Z
dc.date.issued
2018-06
dc.identifier.citation
Rodríguez Hernández, Beatriz; Ondarse Alvarez, Dianelys; Oldani, Andres Nicolas; Martinez Mesa, Aliezer; Uranga Pina, Llinersy; et al.; Modification of Optical Properties and Excited-State Dynamics by Linearizing Cyclic Paraphenylene Chromophores; American Chemical Society; Journal of Physical Chemistry C; 122; 29; 6-2018; 16639-16648
dc.identifier.issn
1932-7447
dc.identifier.uri
http://hdl.handle.net/11336/117583
dc.description.abstract
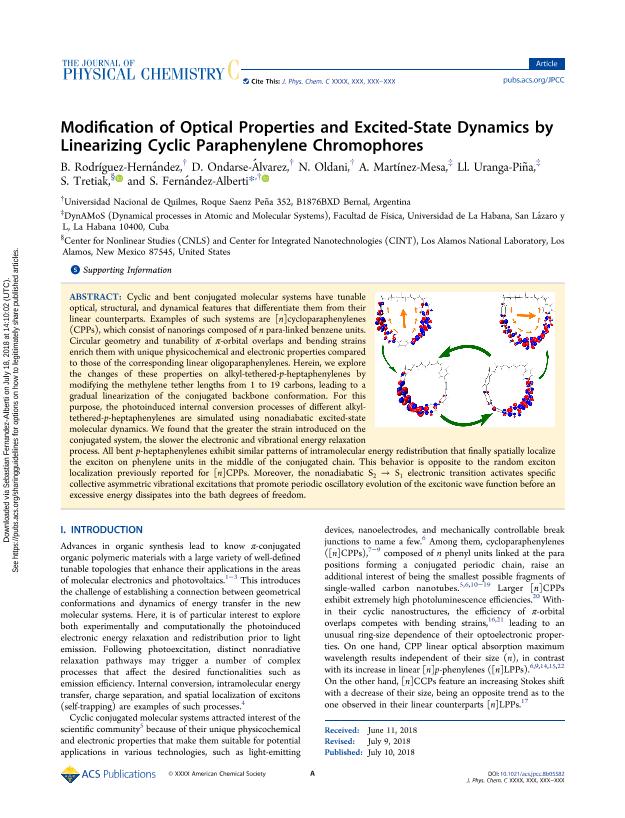
Cyclic and bent conjugated molecular systems have tunable optical, structural, and dynamical features that differentiate them from their linear counterparts. Examples of such systems are [n]cycloparaphenylenes (CPPs), which consist of nanorings composed of n para-linked benzene units. Circular geometry and tunability of π-orbital overlaps and bending strains enrich them with unique physicochemical and electronic properties compared to those of the corresponding linear oligoparaphenylenes. Herein, we explore the changes of these properties on alkyl-tethered-p-heptaphenylenes by modifying the methylene tether lengths from 1 to 19 carbons, leading to a gradual linearization of the conjugated backbone conformation. For this purpose, the photoinduced internal conversion processes of different alkyl-tethered-p-heptaphenylenes are simulated using nonadiabatic excited-state molecular dynamics. We found that the greater the strain introduced on the conjugated system, the slower the electronic and vibrational energy relaxation process. All bent p-heptaphenylenes exhibit similar patterns of intramolecular energy redistribution that finally spatially localize the exciton on phenylene units in the middle of the conjugated chain. This behavior is opposite to the random exciton localization previously reported for [n]CPPs. Moreover, the nonadiabatic S2 → S1 electronic transition activates specific collective asymmetric vibrational excitations that promote periodic oscillatory evolution of the excitonic wave function before an excessive energy dissipates into the bath degrees of freedom.
dc.format
application/pdf
dc.language.iso
eng
dc.publisher
American Chemical Society

dc.rights
info:eu-repo/semantics/openAccess
dc.rights.uri
https://creativecommons.org/licenses/by-nc-sa/2.5/ar/
dc.subject
nanorings
dc.subject
nonadiabatic dynamics
dc.subject
exited states
dc.subject.classification
Físico-Química, Ciencia de los Polímeros, Electroquímica

dc.subject.classification
Ciencias Químicas

dc.subject.classification
CIENCIAS NATURALES Y EXACTAS

dc.title
Modification of Optical Properties and Excited-State Dynamics by Linearizing Cyclic Paraphenylene Chromophores
dc.type
info:eu-repo/semantics/article
dc.type
info:ar-repo/semantics/artículo
dc.type
info:eu-repo/semantics/publishedVersion
dc.date.updated
2020-11-02T17:06:41Z
dc.journal.volume
122
dc.journal.number
29
dc.journal.pagination
16639-16648
dc.journal.pais
Estados Unidos

dc.journal.ciudad
Washington
dc.description.fil
Fil: Rodríguez Hernández, Beatriz. Universidad Nacional de Quilmes. Departamento de Ciencia y Tecnología; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Ondarse Alvarez, Dianelys. Universidad Nacional de Quilmes. Departamento de Ciencia y Tecnología; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Oldani, Andres Nicolas. Universidad Nacional de Quilmes. Departamento de Ciencia y Tecnología; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Martinez Mesa, Aliezer. Universidad de la Habana. Facultad de Física; Cuba. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.description.fil
Fil: Uranga Pina, Llinersy. Universidad de la Habana. Facultad de Física; Cuba
dc.description.fil
Fil: Tretiak, Sergei. Los Alamos National Laboratory; Estados Unidos
dc.description.fil
Fil: Fernández Alberti, Sebastián. Universidad Nacional de Quilmes. Departamento de Ciencia y Tecnología; Argentina. Consejo Nacional de Investigaciones Científicas y Técnicas; Argentina
dc.journal.title
Journal of Physical Chemistry C

dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/url/http://pubs.acs.org/doi/10.1021/acs.jpcc.8b05582
dc.relation.alternativeid
info:eu-repo/semantics/altIdentifier/doi/https://doi.org/10.1021/acs.jpcc.8b05582
Archivos asociados