Artículo
Scanning Electrochemical Microscopy #54. Application To The Study Of Heterogeneous Catalytic ReactionsHydrogen Peroxide Decomposition
Fecha de publicación:
05/2005
Editorial:
American Chemical Society
Revista:
Journal of Physical Chemistry B
ISSN:
1520-6106
Idioma:
Inglés
Tipo de recurso:
Artículo publicado
Clasificación temática:
Resumen
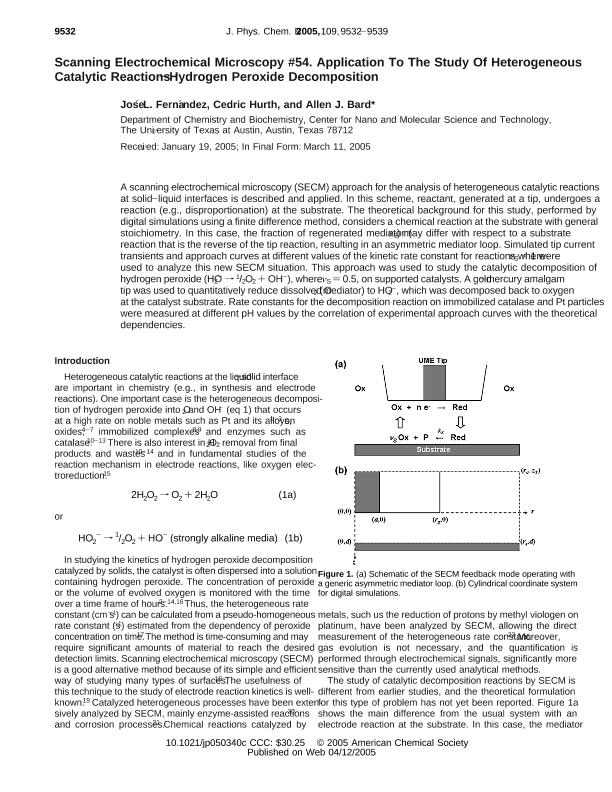
A scanning electrochemical microscopy (SECM) approach for the analysis of heterogeneous catalytic-reactions at solid-liquid interfaces is described and applied. In this scheme reactant, generated at a tip, undergoes a reaction, e.g. disproportionation, at the substrate. The theoretical background for this study, performed by digital simulations using a finite difference method, considers a chemical reaction at the substrate with general stoichiometry. In this case the fraction of regenerated mediator (nS) may differ with respect to a substrate reaction that is the reverse of the tip reaction, resulting in an asymmetric mediator loop. Simulated tip current transients and approach curves at different values of the kinetic rate constant for reactions where nS < 1 were used to analyze this new SECM situation. This approach was used to study the catalytic decomposition of hydrogen peroxide (HO2- ® 1/2O2 + OH-), where nS = 0.5, on supported catalysts. A gold-mercury amalgam tip was used to quantitatively reduce dissolved O2 (mediator) to HO2-, which was decomposed back to oxygen at the catalyst substrate. Rate constants for the decomposition reaction on immobilized catalase and Pt particles were measured at different pH values by correlation of experimental approach curves with the theoretical dependencies.
Archivos asociados
Licencia
Identificadores
Colecciones
Articulos(CCT - SANTA FE)
Articulos de CTRO.CIENTIFICO TECNOL.CONICET - SANTA FE
Articulos de CTRO.CIENTIFICO TECNOL.CONICET - SANTA FE
Citación
Fernandez, Jose Luis; Hurth, Cedric; Bard, Allen; Scanning Electrochemical Microscopy #54. Application To The Study Of Heterogeneous Catalytic ReactionsHydrogen Peroxide Decomposition; American Chemical Society; Journal of Physical Chemistry B; 109; 19; 5-2005; 9532-9539
Compartir
Altmétricas